Scars are not signs of weakness, they are signs of survival and endurance.�� �� Rodney A. Winters
The stock of a one time 'Busted IPO' and 'Tier 4' biotech concern continued its impressive rally last week and is up again Monday in early trade.
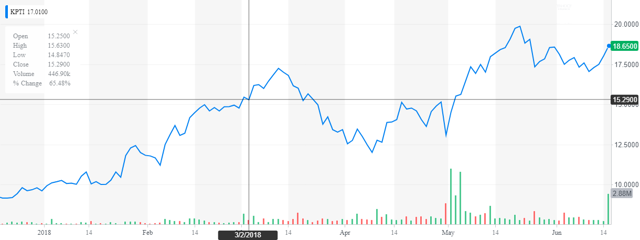
The stock is up more than 80% over the past six months as can be seen from the chart above. Given this, it is time to revisit this name and outline a potential Buy-Write option strategy in the paragraphs below.
Company Overview:
Karyopharm Therapeutics (KPTI) is Massachusetts-based clinical-stage pharmaceutical company just outside Boston. The firm is focused on discovering, developing, and commercializing novel first-in-class drugs directed against nuclear transport and related targets for the treatment of cancer and other major diseases. Karyopharm Therapeutics has a market capitalization of just north of $1.1 billion and trades just over $19.00 a share.
Pipeline:
Source: Company Website
Karyopharm's main drug candidate is selinexor which has received Fast Track status for the treatment of patients with multiple myeloma who have received at least three prior lines of therapy.
Selinexor works to transiently bind to XPO1 transporter proteins, so that tumor suppressor proteins can remain in the nucleus of a cell. Malignant cells over-produce XPO1 which results in tumor suppressor proteins, as well as other beneficial proteins, being transported out of the nucleus. It��s essential that tumor suppressor proteins stay in the nucleus because their function is highly dependent upon their location within the cell. Tumor suppressor proteins are an integral part of the body��s natural defense mechanism in identifying and preventing cancer.
As can be seen by clicking here, the compound has numerous trial readouts scheduled over the next two years.
Recent Events Bolstering Shares:
In late May, the company reached an agreement with China based Antegene for the exclusive development and commercialization of four Karyopharm candidates including selinexor that only applies to various regions of Asia, including China obviously. As part of the deal Karyopharm received a $12 million upfront payout. It also can receive up to $150 million in milestones, tiered double-digit royalties for selinexor and eltanexor and tiered single-to-double-digit royalties for the other two candidates in its pipeline.
Late this week the company presented encouraging trial results from its Phase 1/2 trial 'STOMP' at the big EHA conference in Stockholm. This study is for lead drug selinexor + dexamethasone, combined with Velcade, Pomalyst or Darzalex, in previously treated patients with multiple myeloma.
Analyst Commentary & Balance Sheet:
The current median analyst price target on KPTI is just north of $24.00 a share currently. After STOMP results were presented, H.C. Wainwright reiterated their Buy rating and Street high price target of $30 on Karyopharm with the following color on Friday
We believe this data bodes well for the ongoing Phase 3 BOSTON trial, for which top-line data is expected in 2019. We note that standard Vd regimens (control arm of BOSTON) have an ORR of 60-65% and PFS of 7-9 months according to previous studies. More positive data in the selinexor in the STOMP SDd arm. In the combination selinexor, Darzalex, and low dose dex (SDd) arm evaluating MM patients who received at least three prior lines of therapy, including a PI and an immunomodulatory drug (IMiD), or patients with MM refractory to both a PI and an IMiD, the ORR was 74% (n=19) with an ORR of 82% in Darzalex na茂ve patients (n=17) in evaluable patients as of June 5, 2018. This is relatively in line with prior data, as of for eight Darzalex na茂ve patients, with an ORR of 88%.��
The company ended the first quarter of 2018 with just over $140 million in cash on the books. Karyopharm then did a secondary offering in early May that was well-received and that raised a tad over $150 million in proceeds. The company has stated this will fund all operations through the third quarter of 2019. Karyopharma anticipates filing for accelerated approval of selinexor in multiple myeloma during 2018 with the FDA as well as marketing authorization in Europe for the same indication by the end of this year.
Verdict:
Obviously we liked the risk/reward on Karyopharm better at ~$11 in September when it was added to the model portfolio of the Busted IPO Forum. However, the company has definable potential catalysts on the horizon. Near term funding issues have also been resolved and the shares enjoy solid analyst support. On a longer term basis, slow accumulation to acquire a small stake in Karyopharm within a well-diversified biotech portfolio still seems warranted.
Option Strategy:
An alternative way to accumulate an initial stake or to increase exposure to KPTI is via a Buy-Write order. Using the November $20 call strikes, fashion a Buy-Write order with a net debit of between $16 to $16.30 a share range (net stock price - option premium). This mitigates some downside risk and sets up a more than solid potential return for its five month 'hold' period.
The employed are punished by having to do what they do not love. The self-employed are punished by the opposite.�� �� Mokokoma Mokhonoana
Author's note: If you would like to get these types of articles as soon as they are published, just become a real-time follower of the Busted IPO Forum by clicking here, hitting the big orange "Follow" button, and selecting the "real-time alerts" option.
Disclosure: I am/we are long kpti.
I wrote this article myself, and it expresses my own opinions. I am not receiving compensation for it (other than from Seeking Alpha). I have no business relationship with any company whose stock is mentioned in this article.
No comments:
Post a Comment